Identifying the Additive and Synergistic Effects of Combination Immunotherapy Treatments in a Renal Cell Cancer Patient Derived Xenograft Model
A major challenge in oncology is the identification of effective combinations of immunotherapies with other anti-cancer therapies in addition to biomarkers predictive of response. Traditionally, only one potential combination can be examined per mouse in a systemic dosing study, and large cohorts are needed to draw statistically significant conclusions on its efficacy. The different cellular pathways modulated by a drug combination will likely manifest in a pattern of protein and gene expression as indicators of tumor response as opposed to the change in expression of a single gene or protein. A systematic method to screen many potential combination therapies and measure the intratumor molecular response in a small animal cohort has remained elusive until now.

Clear cell renal carcinoma (CCRC) is the most common type of kidney cancer, accounting for approximately 75% of all renal cell carcinomas(1) and is characterized by the presence of clear cells in the tumor. Clear cell renal carcinoma primarily affects adults, with the average age of diagnosis being around 60 years and men experience the disease at a rate higher than women in a ratio of 2:1. We chose CCRC as our target indication for this study since combination therapies have shown significant advances in treating later-stage or metastatic clear cell renal carcinoma (2). Combining a tyrosine kinase inhibitor (TKI) with an immune checkpoint inhibitor has demonstrated improved overall survival and response rates compared to monotherapy in clinical trials (3). Additionally, dual immune checkpoint inhibitor therapies, such as nivolumab and ipilimumab, have shown increased efficacy compared ton single-agent immunotherapy in certain patient populations (4).
 Combining different targeted therapies with drugs having complementary mechanisms of action, such as TKIs with mTOR
Combining different targeted therapies with drugs having complementary mechanisms of action, such as TKIs with mTOR
inhibitors or VEGF inhibitors, has been explored to overcome resistance and improve outcomes (5). Although different combinations of therapies have been explored clinically, a pre-clinical multi-factorial approach as described here could be an efficient alternative to find novel, high potency combinations of immunotherapies with chemotherapy and targeted agents, and their related response biomarkers, that could have a higher likelihood of clinical benefit in CCRC treatment. We have utilized an implantable microdevice called the Nanonail™ (Figure 1a) to perform cassette microdosing that measures intratumor drug responses and anti-tumor immunity for 11 agents simultaneously(6). For each of the agents, local tumor response is measured by deep cellular response phenotyping with cyclical immunofluorescence and, in a limited number of samples, by spatial transcriptomics with a targeted gene subset. This approach is combined with systemic administration of an anti-PD1 checkpoint inhibitor (nivolumab) to examine whether local immunogenic cell death (ICD) induced by a given drug potentiates the immunotherapy’s anti-tumor effect and if there are patterns of protein and/or gene expression predictive of tumor response.
 The measurements were performed in a humanized mouse model of renal cancer with a patient derived xe-
The measurements were performed in a humanized mouse model of renal cancer with a patient derived xe-
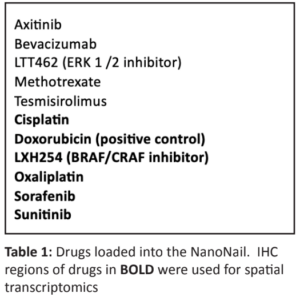
nograft (PDX) RXF488 from a 68-year-old male patient diagnosed with clear cell renal carcinoma. RXF488 was implanted subcutaneously in 30 NSG (immunodeficient) mice (Jackson Laboratories, Inc.) Animals were stratified into six groups of 4-6 mice each and humanization was performed by the intravenous injection of 5×106 human peripheral blood mononuclear cells (PBMC) prior to the first treatment. Systemic anti-PD1 treatment in a clinically relevant dose of 5 mg/kg was applied in the presence and absence of the NanoNail™ and control groups received the microdevice in the presence or absence of PBMCs. Beside the immuno-histological examination and spatial transcriptional profiling of the tumor tissue with the Nanostring CosMx (Nanostring, Inc), flow cytometry (FC) was performed on bone marrow, spleen, and tumor tissue to determine infiltration of human immune cells. Each chemotherapy and targeted agent (Table 1) were individually loaded into a different NanoNail receptacle at a 10 μM concentration.
The microdevice was implanted into each tumor via an 18-gauge biopsy needle and left in place for 5 days, during which each drug diffused into the adjacent tumor tissue to establish a gradient of drug concentration ranging from 10 μM next to the receptacle to 0.1 μM at approximately 0.5 mm into the tissue with an average dose of 1 μM. The NanoNail™ was then recovered with a plug of surrounding tissue after the tumor was excised, fixed in formalin, embedded in paraffin and transversely thin sectioned to reveal the tumor-drug interaction zone for each receptacle. Each thin section was spatially profiled for targeted protein and gene expression and software algorithms were applied that have been specifically developed for analyzing these high complexity data sets.
RESULTS
FC analyses revealed no influence of the treatment on the human immune cells in bone marrow and spleen. The systemic anti-PD1 treatment induced an increase in huCD45+ cells specifically in the tumor tissue only 48 hours after treatment.
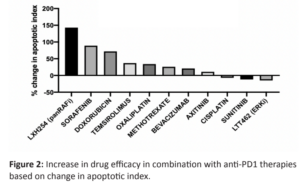
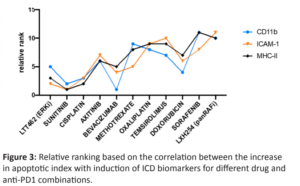
Several agents showed a significant increase in apoptosis induction in the presence of systemically administered Nivolumab with the largest change observed for the panRAF inhibitor LXH254, Sorafenib, and Doxorubicin (Figure 2). This increased efficacy has a strong positive correlation with increased induction of ICD (Figure 3).
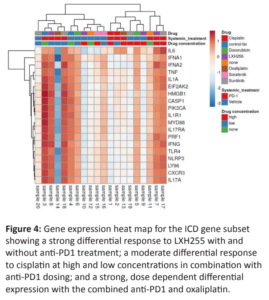
Spatial transcriptomic data was generated with probes targeting 1,825 cancer and immune genes in the same region of IHC measurement. Gene oncology (GO) enrichment analysis with the R umap package (https://rdrr.io/cran/umap/) was performed on genes that were highly up- or down- regulated in comparison to the vehicle control to reveal gene signatures in support of the IHC observations (Figure 4). An up-regulation in the pre-defined immunogenic cell death (ICD) gene subset for LXH254 was observed and a similar yet dose-dependent up-regulation in the same gene subset for Oxaliplatin was found. These observations were concordant with a reactome enrichment analysis on subsets of differentially regulated genes (Figure 5). These data support the hypothesis that LXH254 might play a role as an ICD inducer showing similar transcriptomics patterns than the ICD inducer Oxaliplatin. Cisplatin served as a negative control for ICD.

CONCLUSION
Predicting the efficacy of anticancer drugs in human tumors, either as experimental compounds in preclinical testing or in selection of an optimal therapy for an individual patient, is a major unmet need. The Kibur Medical Nanonail™ directly exposes the tumor in its native microenvironment to a controlled release of up to 20 different drugs or combinations simultaneously, without toxicity risk. Deep phenotypic and genotypic molecular profiling of the tumor molecular response to each drug is a comprehensive description of tumor-drug interactions to reveal new targets, effective combination immunotherapies and biomarkers of efficacy.
The NanoNail™ workflow is a uniquely suited as an efficient approach to implementing the multi-factorial experiments in a single tumor to identify agents that potentiate checkpoint inhibitor activity to increase effectiveness in combination compared with the immunotherapy alone. The approach naturally allows for integration of different spatial measurement modalities like immunohistochemistry and transcriptomics in molecular data sets that show different concordant aspects of pharmacodynamic drug-tumor activity at single cell resolution. The workflow directly supports the FDA Modernization Act 2.0 guidance by maximizing the molecular information on drug-tumor response across different drug agents, combinations and concentrations that results in fewer animals per study (7). In this example, the number of NSG mice consumed decreased by 6-fold with the NanoNail™ workflow.
A related and additional key benefit results from the fact that the scope of drug compounds that can be tested for PD activity early in the development process is greatly expanded because there is no need to optimize each drug compound for systemic delivery. This opens the novel opportunity of early stage, high content in vivo screening of drug compounds, single or in combination, in an intact living tumor for efficacy first and then select those most potent compounds for further development (8). An in vivo efficacy screen greatly shortens the lead selection time and brings higher quality candidates forward since the efficacy screen is in an intact living tumor.
These attributes were utilized in this study to examine whether local immunogenic cell death induced by a given drug potentiates the anti-tumor effect of a systemically delivered checkpoint inhibitor immunotherapy. Protein and gene markers for apoptotic induction and ICD measured in the interaction zone of each of the 11 drugs delivered into the tumor via the NanoNail showed a differential response between each drug and the anti-PD1 checkpoint inhibitor antibody applied compared with the vehicle control. The most significant response was observedwhere genes associated with immunogenic cell death were differentially regulated in the tumor region dosed by LXH254 and Oxaliplatin, respectively, in combination with the systemically delivered anti-PD1. Although preliminary, the measured gene and protein expression profiles could be potentially utilized as indicators of tumor response to the different drug – anti-PD1 combinations.
REFERENCES
1. Garfield K, LaGrange CA. Renal Cell Cancer. [Updated 2022 Aug 1]. In: StatPearls [Internet]. Treasure Island
(FL): StatPearls Publishing; 2023 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470336/
2. Tan et al., J. Immunother Cancer. 2021; 9(7): e002459.
3. Kammerer-Jacquet et al., Int J Mol Sci. 2019 Apr; 20(7):1692.
4. Hsieh, J. et al., Renal cell carcinoma. Nat Rev Dis Primers 3, 17009 (2017). https//doi.org/10.1038/nrdp.2017.9
5. Rossi et al., Curr Oncol Rep. 2021; 23(12): 147.
6. Jonas et al., Sci. Translation Med. 2015;7(284)
7. https://www.congress.gov/bill/117th-congress/senate-bill/5002
8. Jonas et al., J Biomed Nanotechnol. 2016 June ; 12(6): 1297–1302